
April 16, 2024
Assistant Professor Natalie Stanley received a grant from the National Institutes of Health (NIH) to revolutionize diagnostic and predictive capabilities for patients with Alzheimer’s disease.
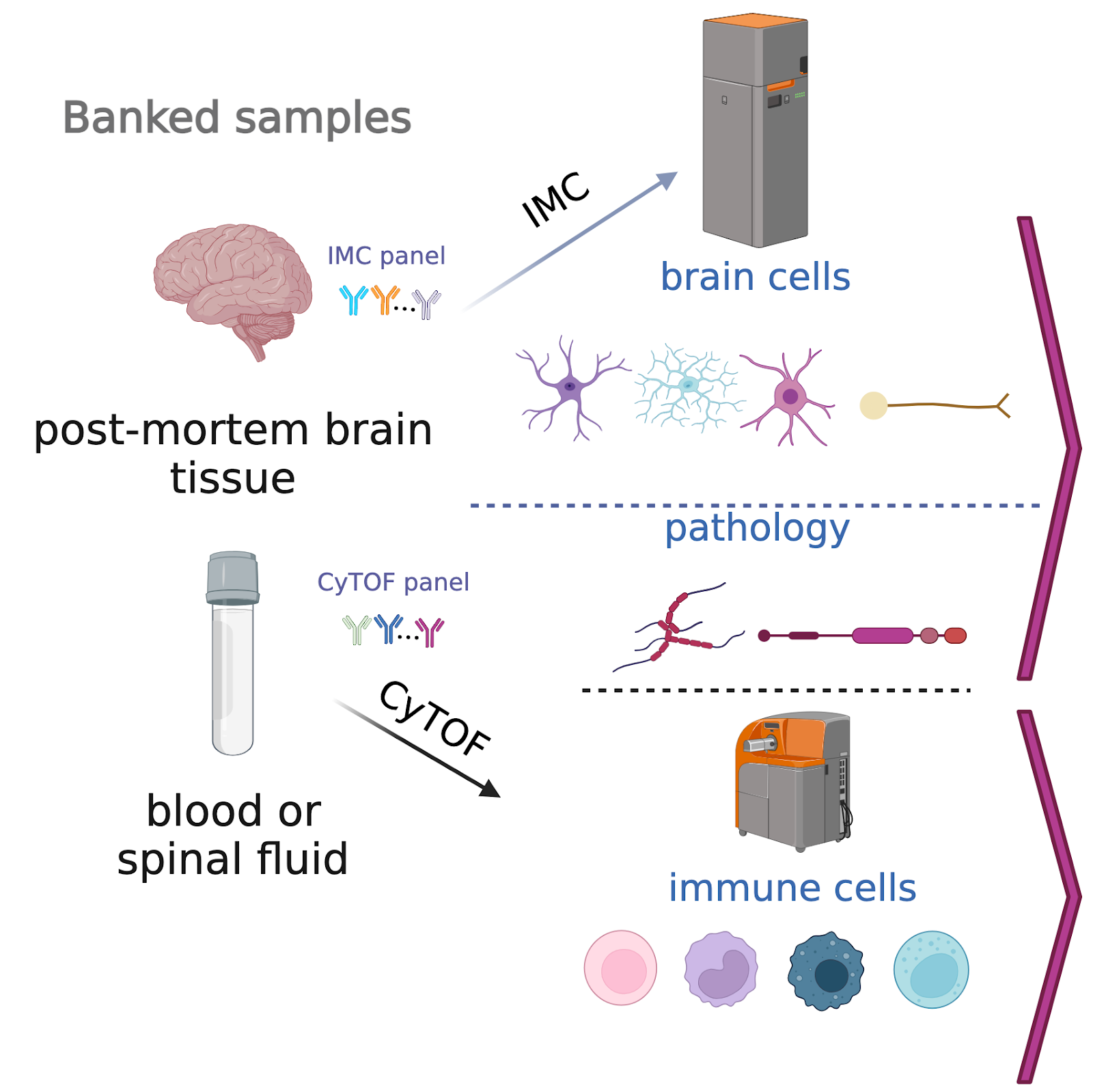
The project, “Spatial signatures of brain health and vulnerability in aging and Alzheimer’s disease,” will leverage imaging mass cytometry (IMC) to allow more rapid, comprehensive, and quantitative detection of particular cell-types in the brain (including neurons, glia, and immune cells) and pathologies that change in response to aging and neurodegeneration. IMC is a spatial single-cell proteomics technique, meaning that it profiles the expression of proteins in individual cells.
The grant will provide $414,486 in direct and indirect funding over two years. Stanley is a co-principal investigator for the project, alongside Todd Cohen from the Department of Neurology, within the UNC School of Medicine.
For decades, the analysis of Alzheimer’s brain pathology has followed largely the same approach, which is a qualitative assessment searching for individual markers of cognitive decline in scans of the brain. Recent developments in IMC have enabled quantitative imaging and evaluation of more than 45 protein markers of interest to efficiently characterize diverse cell-types and their spatial organizational patterns. Large-scale analysis of human and mouse brain samples will provide new and valuable insights into the development of Alzheimer’s disease in the brain. The first step will be to generate a comprehensive brain “signature” of both aging and Alzheimer’s-affected brains. The inclusion of normal aged brains in the study will help delineate the earliest stages of age-related brain changes, when therapeutic interventions can be most effective. Machine learning techniques can then be applied to automate analytical processes, segment brain types, summarize cellular environments, and predict clinical outcomes.
Stanley is excited about the potential impact of the work funded by this grant.
“This project represents a conceptual leap in characterizing diverse cell-types in the brain and how they are spatially organized with respect to each other and pathologies driving neurodegeneration. We are excited to develop new computational approaches to analyze these data efficiently and in an unbiased way at scale to detect early indicators of fast progressing Alzheimer’s disease.” Stanley said.
More information about the project can be found on the NIH website.
Stanley is an assistant professor with appointments in both the Department of Computer Science and the Computational Medicine Program within the UNC School of Medicine. She leads the UNC CompCy Lab (short for Computational Cytometry Lab) at UNC and undertakes research related to single-cell bioinformatics, computational and systems immunology, and algorithms for representing and understanding graph-based data.
